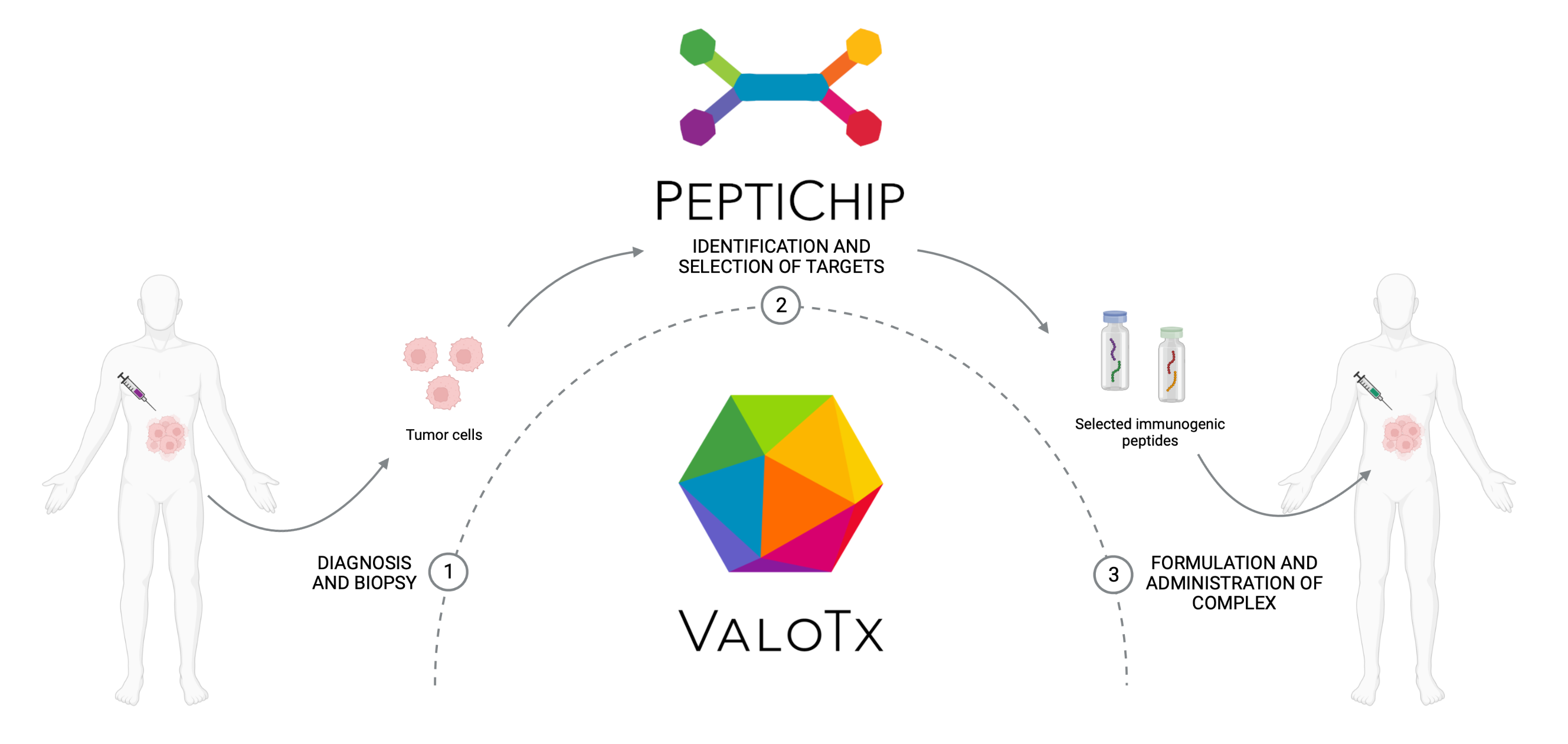
PeptiCHIP is an innovative microchip-based solution that enables rapid, accurate and standardized tumor neoantigen identification from very small tumor biopsies.
Our versatile platform couples a microfluidic immunopurification device with LC/MS-MS analysis and antigen prioritization software. By addressing the technological limitations of existing methods, PeptiCHIP will offer more meaningful and cost-effective neoantigen identification for immunotherapy companies, clinicians and researchers. It is particularly valuable for those looking to discover or validate target antigens that can be used for the clinical development of novel therapies for cancer, as well as infectious, inflammatory, and autoimmune diseases.
Clinical translation of cancer immunotherapies remains elusive since many patients fail to respond to current approaches. To overcome this challenge, immunotherapies must be engineered to target the specific vulnerabilities of each patient’s tumor. This greater degree of personalization can be achieved using neoantigens that are displayed by major histocompatibility complexes (MHC) solely on tumor cells and thus direct the patient’s immune system to recognize the tumors, invoking a strong and highly specific anti-tumor response. Unlocking the full potential of cancer immunotherapies therefore requires more accurate, sample conserving and cost-effective methods to identify the most relevant immunogenic neoantigens.
Currently available neoantigen identification methods have significant limitations by being time-consuming, laborious and expensive, and often unsuitable for analysis of clinical biopsy samples. For instance, the standard ligandome analysis requires a very large amount of tumor material, yet other methods relying on the prediction of MHC-mediated peptide presentation based on genomic information remain inaccurate, impeding the development of novel neoantigen-based immunotherapies.
Together with the software algorithm for ranking of the most promising neoantigens, PeptiCHIP has the potential to overcome these hurdles in the clinical translation of personalized cancer immunotherapies and further facilitate development of novel neoantigen-based immunotherapies. In addition, the device is a valuable tool for basic and pre-clinical research on the immunopeptidome, which is a critical component for understanding the immune system as a whole.
PeptiCHIP offers several advantages for antigen identification:
1. Accuracy
PeptiCHIP purification enables direct determination of neoantigen signatures, precise identification of which is an absolute prerequisite for development of effective personalized immunotherapies
2. Speed
Tumor material is processed on the device at an unparalleled rate - within 1 hour - compared with at least 48 hours for competing technologies
3. Low sample requirement
PeptiCHIP is compatible with clinical tumor biopsy practices as it is able to isolate peptides from significantly smaller samples (~1x106 cells) with minimal loss of the valuable sample material
4. Cost-saving
The running cost for PeptiCHIP-based analyses is significantly lower than that of the less accurate and cumbersome state-of-the-art
5. Convenience
The technology is easy to integrate into existing analysis workflows and does not require specialized training
6. Adaptability
The technology can be applied to basic, pre-clinical, and clinical research, for instance purification of target antigens or validating predicted neoantigens
Relevant publications:
Feola et al. ACS Nano, 15, 10, 15992-16010, https://pubs.acs.org/doi/10.1021/acsnano.1c04371
Chiaro et al. Cancer Immunology Research 2021;9:981–93, https://cancerimmunolres.aacrjournals.org/content/9/8/981

Funded by the European Union (EIC Transition PeptiCHIP # 101112948). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Innovation Council and SMEs Executive Agency (EISMEA). Neither the European Union nor the granting authority can be held responsible for them

